NEW
322Clinical trials
54EPA (Post authorization study)
5MD (Medical device study)
ACTIVE
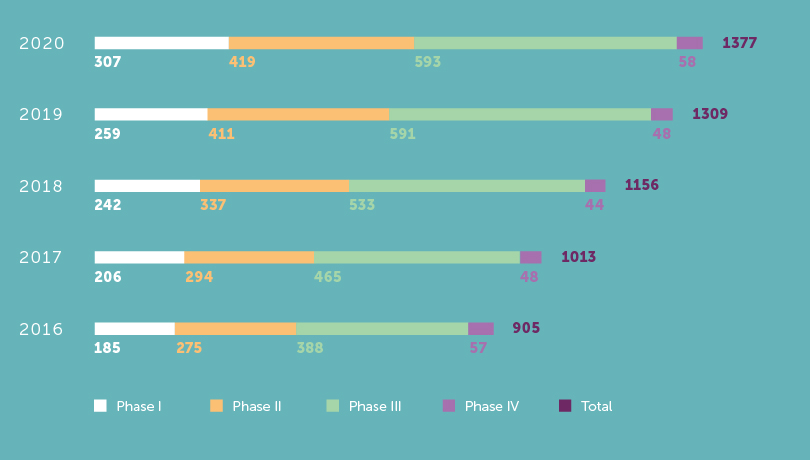
1 377Clinical trials
278EPA (Post authorization study)
40MD (Medical device study)
224Clinical Trials on Rare Diseases
131Clinial Trials on Pediatrics
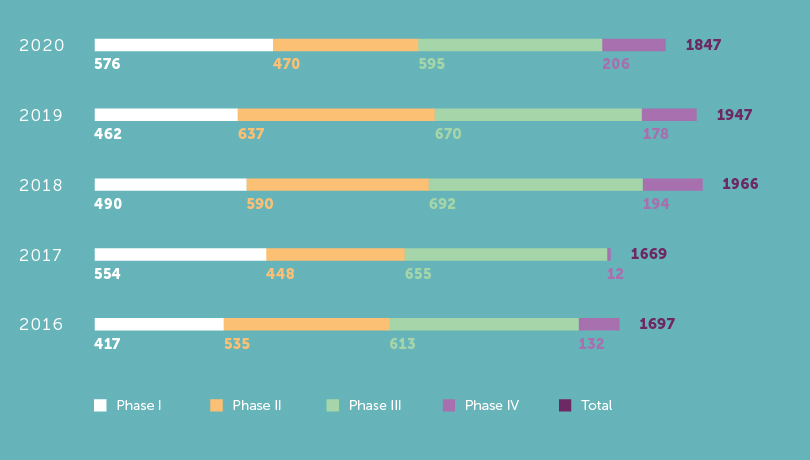
1 847Clinical Trials patient inclusions in 2020
Active Clinical Trials 5-year evolution
Patient inclusions 5-year evolution
Active Clinical Trials by Research Area
60ONCOLOGY
73VASCULAR BIOLOGY AND METABOLISM (VAM)
130NEUROSCIENCES
57INFECTIOUS DISEASES
59DIGESTIVE AND LIVER DISEASES
78IMMUNOMEDIATED DISEASES AND INNOVATIVE THERAPIES
29OBSTETRICS, PEDIATRICS AND GENETICS
10RESEARCH IN SURGERY
865ADULT ONCOLOGY & HEMATHOLOGY (VHIO)
16OTHERS
Billing evolution of clinical trials
| 2016 | 2017 | 2018 | 2019 | 2020 | |
| Phase I-II | 8.59M€ | 9.51M€ | 10.76M€ | 12.64M€ | 12.36M€ |
| Phase III | 5.91M€ | 6.20M€ | 9.12M€ | 8.25M€ | 9.05M€ |
| Phase IV | 0.39M€ | 0.35M € | 0.21M€ | 0.14M€ | 0.19M€ |
| EPA | 0.26M€ | 0.38M€ | 0.24M€ | 0.50M€ | 0.48M€ |
| TOTAL | 15.15M€ | 16.44M€ | 20.33M€ | 21.52M€ | 22.08M€ |
Definitions & methodology
Data is at Campus level
The data includes commercial and academic studies that are registered in the database.
Research Area “Others”: studies of researchers who are not part of any research area of VHIR but belong to a Service of Vall d’Hebron Hospital.
New: studies with signed contract+AEMPS+CEIm.
Active: active+new+closed studies in the year.




